How many moles of phosphorus are present in 80.0 g of phosphorus?
A) 0.387 mol
B) 2.58 mol
C) 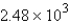 mol
mol
D) 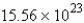 mol
mol
E) none of these
Correct Answer:
Verified
Q100: A compound contains 40.0% carbon, 6.7% hydrogen,
Q101: A compound contains 12.8% C, 2.1% H,
Q102: Calculate the empirical formula of a compound
Q103: Calculate the molecular formula of a compound
Q104: How many molecules of F2 are there
Q106: A compound contains 10.13% C and 89.87%
Q107: A compound has a molar mass of
Q108: The empirical formula for acetic acid is
Q109: How many atoms of phosphorus are present
Q110: The empirical formula of a compound is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents