An atom that has 54 protons, 54 electrons, and 78 neutrons is
A) 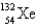
B) 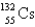
C) 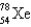
D) 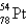
E) 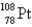
Correct Answer:
Verified
Q63: A certain isotope X2+ contains 37 electrons
Q64: How many neutrons are there in one
Q68: The mass number of an atom equals
A)
Q72: Identify each of the following.
a. The heaviest
Q128: The average mass of a boron atom
Q130: Determine the number of protons and neutrons
Q132: The atom with 69 neutrons and 50
Q144: Which of these are isotopes of hydrogen?
A)
Q145: The number of protons in the nucleus
Q156: Determine the number of protons and the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents