The pressure of an ideal gas is doubled in an isothermal process.The root-mean-square speed of the molecules:
A) does not change
B) increases by a factor of 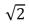
C) decreases by a factor of 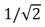
D) increases by a factor of 2
E) decreases by a factor of 1/2
Correct Answer:
Verified
Q27: According to the kinetic theory of gases,
Q28: The root-mean-square speed of molecules in a
Q29: The speeds of 25 molecules are distributed
Q30: What is the relationship between the ideal
Q31: In a system of N gas molecules,
Q33: A quantity of an ideal gas is
Q34: The pressure of an ideal gas is
Q35: The force on the walls of a
Q36: A real gas undergoes a process which
Q37: A real gas is changed slowly from
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents