In the Rutherford model of the hydrogen atom, a proton (mass M, charge Q) is the nucleus and an electron (mass m, charge q) moves around the proton in a circle of radius r.Let k denote the Coulomb force constant (1/4 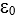 ) and G the universal gravitational constant.The ratio of the electrostatic force to the gravitational force between electron and proton is:
) and G the universal gravitational constant.The ratio of the electrostatic force to the gravitational force between electron and proton is:
A) kQq/GMmr2
B) GQq/kMm
C) kMm/GQq
D) GMm/kQq
E) kQq/GMm
Correct Answer:
Verified
Q31: Two identical conducting spheres A and B
Q32: A particle with charge Q is on
Q33: Two uncharged metal spheres, L and M,
Q34: To what types of electrically charged objects
Q35: Two electrons (e1 and e2)and a
Q37: If excess charge is put on a
Q38: A charge Q is spread uniformly
Q39: Two identical charges, 2.0 m apart, exert
Q40: Two particles have charges Q and -Q
Q41: Which of the following is NOT a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents