The figure shows the energy levels for an electron in a finite potential energy well.If an electron in the n = 2 state absorbs a photon of wavelength 2.0 nm, what happens to the electron? 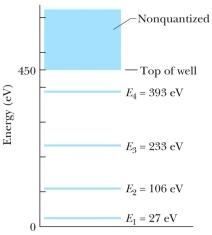
A) It makes a transition to the n = 3 state.
B) It makes a transition to the n = 4 state.
C) It escapes the well with a kinetic energy of 280 eV.
D) It escapes the well with a kinetic energy of 730 eV.
E) Nothing; this photon does not have an energy corresponding to an allowed transition so it is not absorbed.
Correct Answer:
Verified
Q29: The binding energy of an electron in
Q30: An electron is trapped in an infinitely
Q31: A particle in a certain finite potential
Q32: A particle is trapped in a finite
Q33: When a hydrogen atom makes the transition
Q35: Take the potential energy of a hydrogen
Q36: The quantum number n is most closely
Q37: Take the potential energy of a hydrogen
Q38: An electron is trapped in an infinitely
Q39: The Balmer series of hydrogen is important
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents