If the wave function 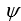 is spherically symmetric then the radial probability density is given by:
is spherically symmetric then the radial probability density is given by:
A) "4 r2 11ef0190_1467_f449_bc85_dd4c7f484907_TB6585_11 "
B) 11ef0190_374b_fdda_bc85_9f14730543b1_TB6585_11
C) 11ef0190_557b_ce2b_bc85_a7ad62d3b83d_TB6585_11
D) 11ef0190_7087_f04c_bc85_cf69b3277e68_TB6585_11
E) 11ef0190_82dc_524d_bc85_b1fcd9b55a0e_TB6585_11
Correct Answer:
Verified
Q35: Take the potential energy of a hydrogen
Q36: The quantum number n is most closely
Q37: Take the potential energy of a hydrogen
Q38: An electron is trapped in an infinitely
Q39: The Balmer series of hydrogen is important
Q40: A particle is confined by finite potential
Q42: The radial probability density for the electron
Q43: If P(r)is the radial probability density for
Q44: Which of the following sets of quantum
Q45: The following image is a dot plot
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents