The magnitude of the orbital magnetic dipole moment 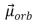 of an atom is ( B is the Bohr magneton, and
of an atom is ( B is the Bohr magneton, and 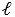 is a positive integer) :
is a positive integer) :
A) " B"
B) " B11ef01a9_ebb2_0dc7_bc85_ad2d297ddd86_TB6585_11"
C) " B
11eab079_97cd_a77a_a51f_8d1202e622a8_TB6585_11"
D) " B (211ef01a9_ebb2_0dc7_bc85_ad2d297ddd86_TB6585_11+1) "
E) " B 11ef01a9_ebb2_0dc7_bc85_ad2d297ddd86_TB6585_112"
Correct Answer:
Verified
Q9: The number of states in a subshell
Q10: An atom is in a state with
Q11: Space quantization means that:
A)space is quantized
B)Lz can
Q12: The Einstein-de Haas experiment showed that:
A)atoms emit
Q13: An electron is in a quantum state
Q15: The number of values of the orbital
Q16: An electron is in a quantum state
Q17: The total number of electron states with
Q18: The magnitude of the orbital angular momentum
Q19: The number of states in a shell
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents