Electrons are in a two-dimensional square potential energy well with sides of length L.The potential energy is infinite at the sides and zero inside.The single-particle energies are given by 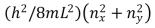 , , where nx and ny are integers.At most the number of electrons that can have energy 8(h2/8mL2) is:
, , where nx and ny are integers.At most the number of electrons that can have energy 8(h2/8mL2) is:
A) 1
B) 2
C) 3
D) 4
E) any number
Correct Answer:
Verified
Q16: An electron is in a quantum state
Q17: The total number of electron states with
Q18: The magnitude of the orbital angular momentum
Q19: The number of states in a shell
Q20: The quantum number ms is most closely
Q22: The quantity sz is related to the
Q23: A magnetic dipole is placed between the
Q24: An electron in an atom is in
Q25: The Stern-Gerlach experiment makes use of:
A)a strong
Q26: The force exerted on a magnetic dipole
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents