Enzymes with a kcat /Km ratio of about 108 M-1s-1 are considered to show optimal catalytic efficiency. Fumarase, which catalyzes the reversible-dehydration reaction 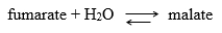 has a ratio of turnover number to the Michaelis-Menten constant, (kcat/Km) of 1.6 × 108 for the substrate fumarate and 3.6 × 107 for the substrate malate. Because the turnover number for both substrates is nearly identical, what factors might be involved that explain the different ratio for the two substrates?
has a ratio of turnover number to the Michaelis-Menten constant, (kcat/Km) of 1.6 × 108 for the substrate fumarate and 3.6 × 107 for the substrate malate. Because the turnover number for both substrates is nearly identical, what factors might be involved that explain the different ratio for the two substrates?
Correct Answer:
Verified
Q88: The enzymatic activity of lysozyme is optimal
Q89: Why does pH affect the activity of
Q90: An enzyme catalyzes the reaction A
Q91: An enzyme follows Michaelis-Menten kinetics. Indicate (with
Q92: Penicillin and related antibiotics contain a
Q94: Chymotrypsin belongs to a group of proteolytic
Q95: Cells can develop a resistance to drugs
Q96: How does the total enzyme concentration affect
Q97: Why is the Lineweaver-Burk (double reciprocal) plot
Q98: Fifteen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents