According to the titration curve to the right, acid A is _____ because the pH _____. 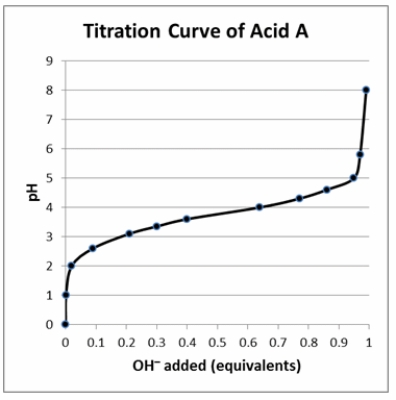
A) weak; resists change when 50% titrated.
B) strong; resists change when 50% titrated.
C) weak; changes dramatically when 100% titrated.
D) strong; changes dramatically when 100% titrated.
E) It cannot be determined from the information given.
Correct Answer:
Verified
Q38: Ice is _ than water because _.
A)
Q39: Which compound would result in the formation
Q40: Which diagram CORRECTLY represent a hydrogen bond?
Q41: The H+ concentration of a solution is
Q42: According to the titration curve above, acid
Q44: According to the Henderson-Hasselbalch equation, when is
Q45: "Proton hopping" essentially means that:
A) an individual
Q46: If a person is suffering from acidosis,
Q47: Milk of magnesia has a pH of
Q48: According to the titration curve to the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents