Ibuprofen is a weak acid with a pKa of 4.9 (shown with the ionizable hydrogen with a star) . It is absorbed through the stomach and the small intestine as a function of polarity-charged and very polar molecules are absorbed slowly; neutral hydrophobic molecules absorb quickly. If the stomach pH is about 1.5 and the small intestine pH is about 6, where (and why) will more ibuprofen be absorbed into the bloodstream? 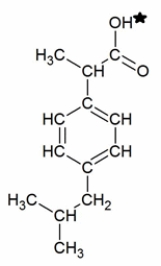
A) More ibuprofen will be absorbed in the small intestine because it will be uncharged due to the pH being greater than the pKa.
B) More ibuprofen will be absorbed in the stomach because it will be uncharged due to the pH being lower than the pKa.
C) More ibuprofen will be absorbed in the small intestine because it will be charged due to the pH being greater than the pKa.
D) More ibuprofen will be absorbed in the stomach because it will be charged due to the pH being lower than the pKa.
E) Ibuprofen will be absorbed equally well in both the stomach and small intestine.
Correct Answer:
Verified
Q53: If the Ka of an acid is
Q54: Which compound acts as a diprotic acid?
A)
Q55: Formic acid is used in the venom
Q56: A 0.6 M solution of a weak
Q57: If the pH of a solution is
Q59: Which statement does NOT describe a strategy
Q60: Distilled white vinegar has a pH of
Q61: Biological buffering systems include:
A) histidine.
B) bicarbonate.
C) phosphate.
D)
Q62: For a weak acid with a pKa
Q63: Give the general Henderson-Hasselbalch equation and indicate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents