Polar molecules cannot easily pass through the cell membrane, but hydrophobic molecules can easily pass through the membrane. The two molecules shown in the diagram both have effects that include raising blood pressure. Comparing two molecules to the right, which statement is TRUE? 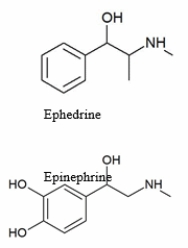
A) Ephedrine can more easily pass through the cell membrane than epinephrine.
B) Epinephrine can more easily pass through the cell membrane than ephedrine.
C) Both epinephrine and ephedrine can pass through the cell membrane equally well.
D) Neither epinephrine nor ephedrine can pass through the cell membrane.
E) None of the statements is true.
Correct Answer:
Verified
Q47: Milk of magnesia has a pH of
Q48: According to the titration curve to the
Q49: The OH- concentration of a solution is
Q50: List the acids in INCREASING order of
Q51: The conjugate base of H2PO4 -1 is:
A)
Q53: If the Ka of an acid is
Q54: Which compound acts as a diprotic acid?
A)
Q55: Formic acid is used in the venom
Q56: A 0.6 M solution of a weak
Q57: If the pH of a solution is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents