The standard reduction potential for ubiquinone (Q or coenzyme Q) is 0.045 V, and the standard reduction potential (E'°) for FAD is -0.219 V. Using these values, show that the oxidation of FADH2 by ubiquinone theoretically liberates enough energy to drive the synthesis of ATP. The Faraday constant, 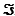 , is 96.48 kJ/V · mol. G'° for ATP synthesis is +30.5 kJ/mol.
, is 96.48 kJ/V · mol. G'° for ATP synthesis is +30.5 kJ/mol.
Correct Answer:
Answered by Quizplus AI
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q64: Under standard conditions, NADH reoxidation by the
Q65: How do the cytosolic and mitochondrial isozymes
Q66: Which phrase BEST describes the role
Q67: Bovine ATP synthase contains 8 c subunits
Q68: Which statement about human mitochondria is TRUE?
A)
Q70: The ATP synthase F1 portion contains many
Q71: Compound X is an inhibitor of mitochondrial
Q72: Mutations in mitochondrial genes do NOT play
Q73: Show the path of electrons from ubiquinone
Q74: As you read and answer this question,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents