Glycerol 3-Phosphate Dehydrogenase Catalyzes the Following Reversible Reaction:
Glycerol 3-Phosphate
Glycerol 3-phosphate dehydrogenase catalyzes the following reversible reaction:
glycerol 3-phosphate + NAD+ NADH + H+ + dihydroxyacetone phosphate
Given the standard reduction potentials below, calculate G'° for the glycerol 3-phosphate dehydrogenase reaction, proceeding from left to right as shown. Show your work. (The Faraday constant, 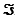 , is 96.48 kJ/V · mol.)
, is 96.48 kJ/V · mol.)
dihydroxyacetone phosphate + 2e- + 2H+ glycerol 3-phosphate
E'° = -0.29 V
NAD+ + H+ + 2e- NADH
E'° = -0.32 V
Correct Answer:
Answered by Quizplus AI
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q95: In glycolysis, the enzyme pyruvate kinase
Q96: If a 0.1 M solution of
Q97: Classify each of the *ed atoms as
Q98: ATP + glucose
Q99: ATP
Q100: What is an oxidation? What is a
Q101: For each pair of ions or compounds
Q103: If
Q104: Lactate dehydrogenase catalyzes the reversible reaction:
pyruvate
Q105: Alcohol dehydrogenase catalyzes the following reversible
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents