Consider the transport of K+ from the blood (where its concentration is about 4 mM) into an erythrocyte that contains 150 mM K+. The transmembrane potential is about 60 mV, inside negative relative to outside. What is the free-energy change for this transport process? (These values may be of use to you: R = 8.315 J/mol . K; T = 298 K; 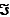 (Faraday constant) = 96,480 J/V; N = 6.022 × 1023/mol.)
(Faraday constant) = 96,480 J/V; N = 6.022 × 1023/mol.)
A) about 5 J/mol
B) about 15 J/mol
C) about 5 kJ/mol
D) about 15 kJ/mol
E) It is impossible to calculate with the information given.
Correct Answer:
Verified
Q33: Which statement does NOT describe a
Q34: Which statement is NOT true about the
Q35: Syndecans are proteoglycans that have glycosaminoglycans attached
Q36: Choline-containing lipids are enriched in the outer
Q37: Consider the transport of glucose into an
Q39: Which statement about facilitated diffusion across a
Q40: ABC transporters are NOT known to facilitate
Q41: FRAP experiments are generally used in membrane
Q42: Bacterial cells change the fluidity of their
Q43: Membrane lipids in tissue samples obtained from
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents