In an experiment,0.243 g of magnesium reacts to give 0.403 g magnesium oxide.Using the conservation of mass law,predict the mass of reacting oxygen gas. 2 Mg(s) + O₂(g) 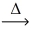 2 MgO(s)
2 MgO(s)
A) 0.080 g
B) 0.160 g
C) 0.320 g
D) 0.646 g
E) 1.292 g
Correct Answer:
Verified
Q16: What term refers to the mass of
Q17: How many moles of steam,H₂O,react with 1
Q18: What is the term for the substance
Q19: What term refers to the volume occupied
Q20: What is the term for the relationship
Q22: How many moles of iodine vapor react
Q23: How many moles of hydrogen iodide are
Q24: In an experiment,0.327 g of zinc metal
Q25: How many moles of water react with
Q26: In an experiment,5.585 g of iron metal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents