How many moles of hydrogen gas react to yield 1.00 mol of hydrogen iodide? __H₂(g) + __I₂(g) 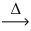 __HI(g)
__HI(g)
A) 0.500 mol
B) 1.00 mol
C) 2.00 mol
D) 4.00 mol
E) none of the above
Correct Answer:
Verified
Q35: How many moles of lithium metal react
Q36: How many moles of water react with
Q37: How many moles of oxygen gas react
Q38: How many moles of oxygen are produced
Q39: In an experiment,0.197 g of gold metal
Q41: In general,how many unit factors are required
Q42: In general,how many unit factors are required
Q43: Which of the following steps is necessary
Q44: Classify the following type of stoichiometry problem:
Q45: Classify the following type of stoichiometry problem:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents