How many moles of nitrogen dioxide gas are produced from 1.00mol NO? __NO(g) + __O₂(g) 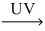 __NO₂(g)
__NO₂(g)
A) 0.250 mol
B) 0.500 mol
C) 1.00 mol
D) 2.00 mol
E) none of the above
Correct Answer:
Verified
Q23: How many moles of hydrogen iodide are
Q24: In an experiment,0.327 g of zinc metal
Q25: How many moles of water react with
Q26: In an experiment,5.585 g of iron metal
Q27: How many moles of water are produced
Q29: In an experiment,0.520 g of chromium metal
Q30: How many moles of oxygen gas react
Q31: How many moles of hydrogen peroxide decompose
Q32: In an experiment,1.201 g of charcoal reacts
Q33: Assuming similar conditions,how many liters of steam,H₂O,react
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents