What is the volume of oxygen gas at STP from the decomposition of 10.8 g of mercuric oxide (216.59 g/mol) ? __HgO(s) 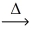 __Hg(l) + __O₂(g)
__Hg(l) + __O₂(g)
A) 0.558 L
B) 1.12 L
C) 2.23 L
D) 52.2 L
E) 209 L
Correct Answer:
Verified
Q57: Classify the following type of stoichiometry problem:
Q58: Which of the following steps is necessary
Q59: How many moles of oxygen gas react
Q60: Classify the following type of stoichiometry problem:
Q61: What is the mass of aluminum metal
Q63: What is the mass of insoluble silver
Q64: What is the mass of copper metal
Q65: What is the volume of oxygen gas
Q66: What is the mass of aluminum metal
Q67: What is the mass of iron(III)bromide (295.55
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents