What is the mass of mercuric oxide (216.59 g/mol) that decomposes to release 0.750L of oxygen gas at STP? __HgO(s) 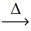 __Hg(l) + __O₂(g)
__Hg(l) + __O₂(g)
A) 0.0388 g
B) 0.155 g
C) 3.63 g
D) 7.25 g
E) 14.5 g
Correct Answer:
Verified
Q65: What is the volume of oxygen gas
Q66: What is the mass of aluminum metal
Q67: What is the mass of iron(III)bromide (295.55
Q68: What is the mass of aluminum metal
Q69: What is the mass of aluminum oxide
Q71: What is the mass of insoluble calcium
Q72: What is the mass of potassium iodide
Q73: What is the mass of silver metal
Q74: What is the mass of sodium phosphate
Q75: In general,how many unit factors are required
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents