What volume of methane gas,CH₄,reacts to give 5.00 mL of carbon dioxide? (Assume temperature and pressure remain constant. )
__CH₄(g) + __O₂(g) 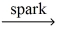 __CO₂(g) + __H₂O(g)
__CO₂(g) + __H₂O(g)
A) 2.50 mL
B) 5.00 mL
C) 10.0 mL
D) 20.0 mL
E) none of the above
Correct Answer:
Verified
Q92: What is the mass of silver chlorate
Q93: What is the volume of carbon dioxide
Q94: What is the mass of aluminum metal
Q95: What volume of oxygen gas reacts with
Q96: What volume of oxygen gas reacts to
Q98: What volume of oxygen gas reacts with
Q99: What is the mass of sodium metal
Q100: What is the volume of hydrogen gas
Q101: Considering the limiting reactant concept,how many moles
Q102: Considering the limiting reactant,what is the volume
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents