Which of the statements listed below is true regarding the following redox reaction occurring in a nonspontaneous electrochemical cell? 3 C(s) + 4 AlCl3(l) 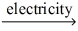 4 Al(l) + 3 CCl4(g)
4 Al(l) + 3 CCl4(g)
A) oxidation half-reaction: Al3+ + 3 e- →Al
B) reduction half-reaction: C + 4 Cl- → CCl4 + 4 e-
C) Cl- is produced at the anode.
D) Al metal is produced at the cathode.
E) none of the above
Correct Answer:
Verified
Q114: Which of the statements listed below is
Q115: Which of the statements listed below is
Q116: Which of the statements listed below is
Q117: Which of the statements listed below is
Q118: Which of the statements listed below is
Q120: Which of the statements listed below is
Q121: Which of the statements listed below is
Q122: Which of the statements listed below is
Q123: Which substance listed below is the weakest
Q124: Which substance listed below is the weakest
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents