Which of the statements listed below is true regarding the following redox reaction occurring in a nonspontaneous electrochemical cell? Cd(s) + Zn(NO₃) 2(aq) 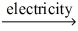 Zn(s) + Cd(NO₃) 2(aq)
Zn(s) + Cd(NO₃) 2(aq)
A) oxidation half-reaction: Cd → Cd₂+ + 2 e-
B) reduction half-reaction: Zn₂+ + 2 e- →Zn
C) Cd₂+ is produced at the anode.
D) Zn metal is produced at the cathode.
E) all of the above
Correct Answer:
Verified
Q125: Given that the following redox reactions go
Q126: Nickel-cadmium batteries are used in rechargeable electronic
Q127: Which of the statements listed below is
Q128: Given that the following redox reactions go
Q129: Which of the statements listed below is
Q131: What are the two sources of energy
Q132: Given that the following redox reactions go
Q133: After balancing the following redox reaction in
Q134: Which of the statements listed below is
Q135: Nickel-cadmium batteries are used in rechargeable electronic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents