If a sample of methane gas,CH₄,reacts completely with oxygen gas to give 1.75 g of water,what STP volume of methane reacted? CH₄(g) + O₂(g) 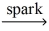 CO₂(g) + H₂O(l)
CO₂(g) + H₂O(l)
A) 0.703 L
B) 1.09 L
C) 1.41 L
D) 2.18 L
E) 4.36 L
Correct Answer:
Verified
Q56: What are the units of the unknown
Q57: If 1.12 L of HCl gas at
Q58: If 5.00 g of ammonia,NH₃,is dissolved in
Q59: If 1.12 L of HCl gas at
Q60: If 5.00 g of ammonia,NH₃,is dissolved in
Q62: Given the three-step Contact process for manufacturing
Q63: Given the three-step "blast furnace" process that
Q64: Given the three-step "blast furnace" process that
Q65: If a sample of ammonia gas,NH₃,reacts with
Q66: Given that 50.0 mL of 0.125 M
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents