When acetic acid is dissolved in water which of the following is true of the equilibrium which is established as represented below? 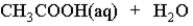

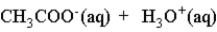
A) It lies very far to the left.
B) It lies slightly to the left.
C) It lies very far to the right.
D) It lies slightly to the right.
Correct Answer:
Verified
Q42: Which of the following acids has the
Q47: Which of the following bases has the
Q59: Which of the following statements best describes
Q61: When comparing the strength of two weak
Q62: Hydrocyanic acid, HCN, is a weaker acid
Q64: When comparing two acids which of the
Q65: Consider the following hypothetical acid-base reaction. A(aq)
Q66: What are the two acids in the
Q67: When comparing the strength of two weak
Q90: The pKa of hydrocyanic acid is 9.31.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents