What is the effect of adding H2O(l) to a container in which the reaction has reached equilibrium? 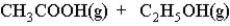

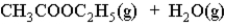
A) The reaction will shift left to right.
B) The reaction will shift right to left.
C) There will be no effect.
D) What happens depends on the temperature.
Correct Answer:
Verified
Q93: Which of the following describes the effect
Q94: For the exothermic reaction Q95: If the exothermic reaction Q96: Which of the following is the equilibrium Q97: Consider the following energy diagram for a Q99: Which of the following describes the effect Q100: For the exothermic reaction Q101: Consider the following graph. Q102: Consider the following graph. Q103: Consider the following graph. Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()
![]()
![]()

