Consider the following energy diagram for a reaction. 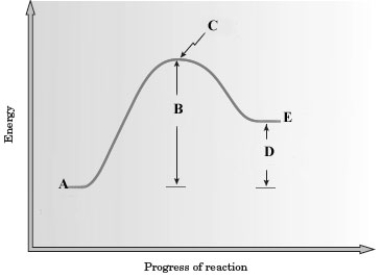
-Which of the following correctly represents this reaction?
A) The energy of the products is less than that of the reactants so the reaction is endothermic.
B) The energy of the products is greater than that of the reactants so the reaction is endothermic.
C) The energy of the products is less than that of the reactants so the reaction is exothermic.
D) The energy of the products is greater than that of the reactants so the reaction is exothermic.
Correct Answer:
Verified
Q77: Which of the following could be the
Q78: Which of the following will change the
Q79: Which of the following will change the
Q80: Which of the following is generally not
Q81: Which of the following is true of
Q83: Consider the following energy diagram for a
Q84: A particular reaction has an equilibrium constant
Q85: When considering the effect of a catalyst
Q86: If the endothermic reaction Q87: Consider the following energy diagram for a![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents