Consider the following phase diagram of water. The temperature and pressure scales are greatly reduced (and are non-linear) 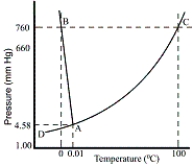
-Consider the model shown below. Each sphere represents an atom.  To which of the following classification might this substance belong?
To which of the following classification might this substance belong?
A) ionic solid
B) amorphous solid
C) molecular solid
D) metallic solid
Correct Answer:
Verified
Q101: The normal boiling point of acetic acid
Q116: Which of the following is an example
Q117: Which of the following phase changes does
Q119: Which of the following pieces of data
Q120: Fullerenes are examples of which of the
Q122: Consider the following phase diagram of water.
Q123: Consider the following phase diagram of water.
Q124: Consider the following heating curve for the
Q125: Consider the following heating curve for the
Q126: Consider the following phase diagram of water.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents