Consider the following Lewis structure. 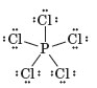
-Which of the following correctly characterizes this compound?
A) All atoms obey the octet rule.
B) 40 valence electrons are represented.
C) The central atom is from period 4.
D) Contains a metalloid and a nonmetal.
E) None of these are true of this compound.
Correct Answer:
Verified
Q102: Which of the following molecules is best
Q117: Which of the following statements about nitric
Q118: Which of the following is true of
Q119: Which of the following approximates the H-N-H
Q120: Which of the following is true in
Q122: Which of the following molecules is polar?
A)
Q123: Which of the following molecules is(are) polar?
A)
Q124: Which of the following molecules is(are) polar?
A)
Q125: A student discussing bond polarity and molecular
Q126: Which of the following molecules is(are) polar?
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents