A beaker contains two solutions of glucose dissolved in water. The two solutions have different concentrations (measured by molarity, M) and are separated by a membrane that is permeable to water, but not to glucose. 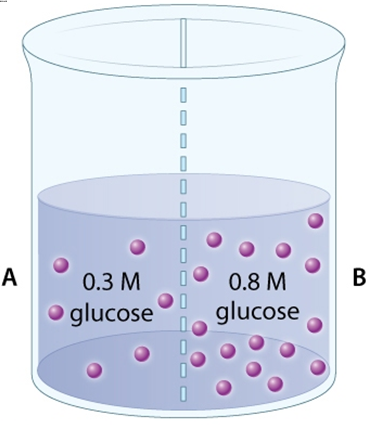
Which of the following statements is TRUE?
A) There will be net movement of glucose from B to A.
B) The volume will increase in side B of the beaker.
C) There will be net movement of water from B to A.
D) The system will not reach equilibrium.
Correct Answer:
Verified
Q58: Some drugs are now being delivered into
Q59: Suppose you are studying the transport of
Q60: Some diseases result from defective transport across
Q61: The movement of water into, or out
Q62: The random movement of molecules is referred
Q64: Which of the following represents how easily
Q65: The MOST abundant organic molecule in nature
Q66: What factors are required for net movement
Q67: In certain cells, a transport protein moves
Q68: Which of the following have cell walls?
A)plant
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents