The compound HA is an acid that is soluble in water. Which of the "beakers" below shows HA behaving as a weak acid in water?
A) 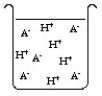
B) 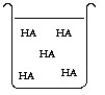
C) 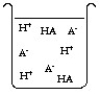
D) Both A and C are weak acids in water.
Correct Answer:
Verified
Q3: Which of the following compounds is not
Q4: Which of the following acids produces more
Q5: Which of the following compounds is a
Q6: Which of the following compounds is a
Q7: Which of the following acids can produce
Q9: Which of the following acids is a
Q10: Which of the following acids is the
Q11: Which of the following solutions would you
Q12: Which of the following compounds is an
Q13: Which of the following compounds is a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents