Which of the following conjugate pairs would form a good buffer?
A) HCl/ 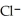
B) 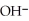 /
/ 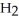 O
O
C) 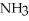 /
/  +
+
D) 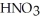 /
/ 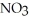 -
-
Correct Answer:
Verified
Q73: Carbonic acid, phosphoric acid, and sulfuric acid
Q74: An electrolyte is a substance that, when
Q75: The conjugate base of ammonia is _.
A)
Q76: Solid sodium chloride is a strong electrolyte.
Q77: Water can serve both as a Bronsted-Lowry
Q79: Hydrochloric, hypochlorous and hydrobromic acids produce only
Q80: Arrhenius' definition of a base is an
Q81: A solution of HCl/ Cl- can act
Q82: What were the defining characteristics of bases
Q83: What is the pH of an aqueous
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents