The compound HCl is considered a Bronsted-Lowry base because it increases the concentration of 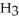
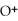 when it dissociates in water.
when it dissociates in water.
Correct Answer:
Verified
Q63: A concentrated solution of a non-electrolyte such
Q64: Sucrose (table sugar) is a strong electrolyte.
Q65: CH₃CO₂H has four acidic hydrogens.
Q66: Sodium chloride (NaCl) is considered an electrolyte
Q67: Sulfuric acid is a diprotic acid.
Q69: A baking soda solution is a strong
Q70: An aqueous solution of HCl is called
Q71: Deionized water can conduct electricity.
Q72: Acetic acid is a diprotic acid.
Q73: Carbonic acid, phosphoric acid, and sulfuric acid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents