True/False
The carbonate ( 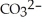 )/bicarbonate (
)/bicarbonate ( 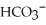 ) pair represents a base and its conjugate acid.
) pair represents a base and its conjugate acid.
Correct Answer:
Verified
Related Questions
Q91: If the pOH of a solution is
Q92: Milk is slightly basic.
Q93: Write down the chemical equation for the
Q94: Approximately one out of every 555 million
Q95: In a solution of a strong monoprotic
Q97: An aqueous solution of pOH 5 is
Q98: What were the three main defining characteristics
Q99: PH₃ cannot serve as a Bronsted-Lowry base.
Q100: H₂PO₄- is the conjugate acid of PO₄2-.
Q101: Identify each of the substances as an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents