The following chemical reaction has reached equilibrium. 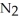
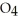 (g)
(g) 
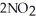 (g) Calculate the equilibrium constant, Keq, if the concentrations of
(g) Calculate the equilibrium constant, Keq, if the concentrations of 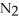
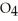 and
and 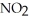 are 0.0238 M and 0.196 M, respectively.
are 0.0238 M and 0.196 M, respectively.
A) 8.34
B) 346
C) 0.621
D) 1.61
Correct Answer:
Verified
Q9: The equilibrium constant is equal to which
Q10: The equilibrium constant expression for the reaction
Q11: Which of the following statements is true
Q12: Increasing the pressure will _. 2 HCl
Q13: The equilibrium constant expression for the reaction
Q15: The following chemical reaction has reached equilibrium.
Q16: Which of the following statements best describes
Q17: When a reaction shows one arrow pointing
Q18: If the value of the equilibrium constant
Q19: If the value of the equilibrium constant
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents