Oxygen (O₂) and ozone (O₃) coexist in the stratosphere and are in equilibrium with one another: 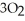

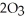 a) Assuming that no other factors affect this equilibrium, if ozone (
a) Assuming that no other factors affect this equilibrium, if ozone ( 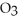 ) is being "depleted" (somehow removed from the reaction system entirely), what is the direction of the equilibrium shift?
) is being "depleted" (somehow removed from the reaction system entirely), what is the direction of the equilibrium shift?
b) What must be happening to the concentration of oxygen ( 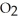 ) as a result of the equilibrium shift predicted in part a?
) as a result of the equilibrium shift predicted in part a?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q86: No reaction exists in which 100% of
Q87: Adding extra nickel will shift the equilibrium
Q88: Changing the temperature of a reaction will
Q89: The equilibrium condition of a chemical reaction
Q90: Write an expression for the equilibrium constant
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents