Predict the effect of increasing pressure in each of the reactions by choosing the appropriate direction shift.
-2 SO₃ (g) + 2 CL₂ (g) 
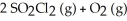
A) no effect
B) right (→)
C) left (←)
Correct Answer:
Verified
Q105: For the following endothermic reaction:
C (s) +
Q106: Identify each of the equilibrium constant values
Q107: Predict the effect of increasing pressure in
Q108: Predict the direction in which the reaction
Q109: Predict the effect of increasing pressure in
Q111: Match each reaction and its equilibrium concentrations
Q112: Identify each of the equilibrium constant values
Q113: Identify each of the equilibrium constant values
Q114: Match each reaction and its equilibrium concentrations
Q115: Predict the effect of increasing pressure in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents