A 2.00 g sample of a non-electrolyte (molar mass = 118) is dissolved in 55.0 g of benzene (Kf of benzene is 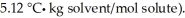 What is the freezing point depression of the solution?
What is the freezing point depression of the solution?
A) 1.58 °C
B) 3.16 °C
C) 0.79 °C
D) 2.33 °C
Correct Answer:
Verified
Q37: To prepare 500. mL of 0.5 M
Q38: To prepare 100 mL of 0.4 M
Q39: 10.0 mL of 0.50 M sulfuric acid
Q40: 500 mL of a 0.4 M calcium
Q41: A solid solution is a solution of
Q43: Transparent solutions are examples of homogeneous matter.
Q44: An impurity _ the boiling point of
Q45: Vinegar is a liquid solution where acetic
Q46: If the empirical formula is C₄H₃N and
Q47: Phenolphthalein turns _ when the solution is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents