For the following reaction, explain how you will use IR spectroscopy to monitor the progress of the reaction. 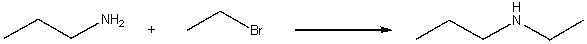
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q65: In mass spectrometry, the tallest peak is
Q71: Mass spectrometry is primarily used to determine:
A)
Q72: Which of the following is not true
Q73: Which of the m/z values correspond to
Q75: The separation of ions in the mass
Q77: Which of the following is initially produced
Q78: Predict the product for the following reaction
Q80: Predict the product for the following reaction
Q86: Which of the following m/z values corresponds
Q107: Which of the following is not a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents