The free radical polymerization of styrene with benzoyl peroxide yields a polymer that has repeat units arranged primarily in a 'head-to-tail' arrangement. This means that the phenyl group primarily ends up placed at alternating carbon atoms along the chain. Use correct arrow formalism to show why this arrangement is preferred over a 'head-to-head' or 'tail-to-tail' arrangement. 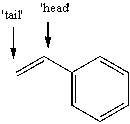
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q63: Which term best describes the process shown
Q64: Your textbook mentions that the free radical
Q65: One possible product of thermal cracking of
Q66: Predict the major product(s) of the reaction
Q67: Predict the product(s) of the following reaction:
Q69: Azobisisobutyronitrile (AIBN) is commonly used as a
Q71: Predict the product(s) of the following reaction:
Q72: Which intermediate leads to the major product
Q73: Use correct arrow formalism to show termination
Q96: Which monomer is used for the synthesis
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents