Later in the course, we will compare the halogenation of differently substituted carbons, comparing reactions like the ones below. Which of the following reactions has a more exothermic heat of reaction ( Ho) ? 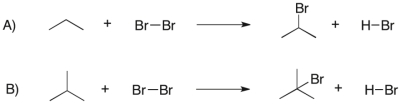
A) Reaction A has a more exothermic heat of reaction ( Ho)
B) Reaction B has a more exothermic heat of reaction ( Ho)
C) Both reactions have the same heat of reaction ( Ho)
Correct Answer:
Verified
Q8: Later in the course, we will
Q9: Estimate the enthalpy change of the following
Q10: Predict the sign of
Q11: Predict the sign of
Q12: Of following reactions, which one(s) would
Q14: Which of the following would you
Q16: Predict the sign of
Q17: Why is the entropy change negative for
Q17: Which of the following is the enthalpy
Q18: What type of bond cleavage does the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents