Consider the following reaction. What patterns of arrow pushing are likely involved in the formation of the product? Would you expect the S for the reaction to be positive or negative? 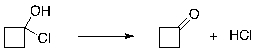
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q98: Draw curved arrows for each step of
Q99: Identify the nucleophilic and electrophilic sites in
Q100: What pattern of curved arrow pushing is
Q101: Draw the mechanism and most likely product
Q102: Draw the most likely structure of the
Q104: Consider the product of the following mechanism.
Q105: Which of the following is the most
Q106: Will the following cation undergo rearrangement?
Q107: Draw the mechanism and most likely product
Q108: Draw the structure of the most likely
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents