How many total lone pairs of electrons are on both oxygen atoms in the following compound? 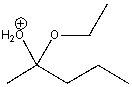
A) one
B) two
C) three
D) four
E) none
Correct Answer:
Verified
Q79: Diazomethane has the molecular formula CH2N2. Draw
Q80: Draw Lewis structure for the following compound.
Q81: The indicated bond in the following compound
Q82: Draw all lone pairs of electrons for
Q83: For the following compound label the bonds
Q85: The indicated bond in the following compound
Q86: Draw all lone pairs of electrons for
Q88: Which of the following is a Haworth
Q89: Which of the following pairs are resonance
Q101: Delocalization of charge over two or more
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents