What is the hybridization state of the nitrogen atom in the following compound? 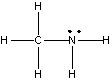
A) sp
B) sp2
C) sp3
D) sp3d
E) s2p
Correct Answer:
Verified
Q84: According to molecular orbital theory the highest
Q89: Constructive interference of waves results in_.
A) a
Q90: According to molecular orbital theory, the destructive
Q91: The difference between valence bond theory and
Q93: According to molecular orbital theory, the constructive
Q95: Which of the bonding type has
Q96: Interaction of the following two atomic orbitals
Q97: Interaction of the following two atomic orbitals
Q98: Destructive interference of waves results in_.
A) a
Q99: Interaction of the following two atomic orbitals
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents