Which of the following compounds have trigonal pyramidal molecular geometry? 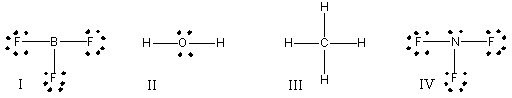
A) I
B) II
C) III
D) IV
E) I & IV
Correct Answer:
Verified
Q133: What is the molecular geometry at the
Q134: What is the molecular geometry at the
Q135: What is the hybridization state of the
Q136: Which of the following compounds have bent
Q137: The carbon and oxygen atoms in
Q139: Rank the indicated C-C bonds in increasing
Q140: Which is the shortest bond in the
Q141: What is the hybridization state and approximate
Q142: What is the approximate bond angle around
Q143: Which compound does not have a linear
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents