Predict the specific rotation of the compound shown. 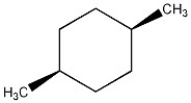
A) It is impossible to predict; it must be determined experimentally.
B) Because both asymmetric centers are R, the compound is dextrorotatory.
C) Because both asymmetric centers are S, the compound is levorotatory.
D) Zero; the compound is achiral.
E) Because this compound represents a racemic mixture, the compound is dextrorotatory.
Correct Answer:
Verified
Q68: If (S)-glyceraldehyde has a specific rotation of
Q69: Calculate the e.e. of a mixture containing
Q72: Which of the following configurations corresponds to
Q74: Captopril is used to treat high blood
Q75: For the structure shown below, draw the
Q77: Label each asymmetric carbon in the compound
Q79: A newly isolated natural product was shown
Q80: Would a 50:50 mixture of (2R,3R)-2,3-dibromobutane and
Q80: Phantasmidine, shown below, is found in
Q81: Draw the Fischer projection of (S)-2-hydroxybutanoic acid,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents