Provide the structure of the ester that would undergo self-condensation to yield the β-ketoester shown below. 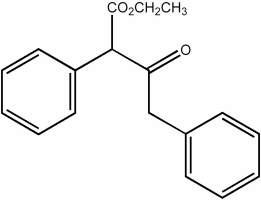
Correct Answer:
Verified
Q56: An enolate attacks an aldehyde and the
Q59: Which set of reagents would best accomplish
Q61: Provide the sequence of steps necessary to
Q63: Predict the pKa unit of the indicated
Q64: What product results when an aldol is
Q65: Provide the structure of the aldol product
Q67: What two molecules were condensed in an
Q67: Which of the following could result from
Q75: What crossed-aldol product results when propanal is
Q80: In theory a poorly planned crossed aldol
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents