Beta-keto acids are unusually unstable and will lose the carboxylate group under certain conditions where both a general acid and base are involved. During this process, CO2 is lost and the original beta-keto acid is converted into a ketone. For the following reaction, show a stepwise mechanism, with electron pushing arrows, in the formation of the products. Make special note of any resonance stabilized intermediates in this reaction. 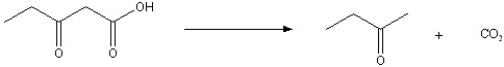
Correct Answer:
Verified
Q87: Give a detailed, stepwise mechanism for the
Q98: What reagent is needed to complete the
Q100: Provide the major organic product of the
Q101: Provide the major organic product for the
Q102: Lithium aluminum hydride reduces carboxylic acids to
Q104: Provide the major organic product of the
Q105: Provide the major organic product of the
Q106: Provide the structure of the major organic
Q115: Which of the following reagents can be
Q116: Esters and amides are most easily made
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents