What kind of molecular orbital (σ, σ*, π, or π*) results when the two atomic orbitals shown below interact in the manner indicated? 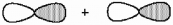
Correct Answer:
Verified
Q15: How many π bonds are present in
Q16: How many carbon-carbon s bonds are present
Q18: Which atomic orbital combination would result in
Q18: Which of the following statements about π
Q19: What kind on molecular orbital (σ, σ*,
Q21: The CCC bond angle in allene (H2CCCH2)
Q23: What two hybrid atomic orbitals overlap to
Q25: How many sp2 hybridized carbon atoms are
Q26: Choose the correct hybridization for the atom
Q34: What is the approximate value of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents