Which of the following line orbital energy diagrams describes the orbital location of valence electrons in an sp3 hybridized carbon atom (consider that SP is a generic notation that could reference either sp, sp2 or sp3 hybrid orbitals) ?
A) 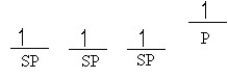
B) 
C) 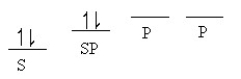
D) 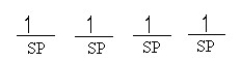
E) 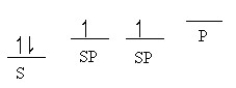
Correct Answer:
Verified
Q26: Choose the correct hybridization for the atom
Q28: What is the approximate value of the
Q28: What two hybrid atomic orbitals overlap to
Q30: Circle the coplanar atoms in 1-ethylcyclopentene shown
Q31: Based on the structure below, what is
Q31: The HCH bond angle in allene (H2CCCH2)
Q34: In the structure below, the sigma bond
Q35: Vildagliptin is a recently released antidiabetic drug
Q36: Complete the structure of methyl azide by
Q37: How many sp3 hybridized carbon atoms are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents